Chemical Manufacturing – Chromium Pollution
The chemical manufacturing sector is expansive and diverse, and is split into six different categories by the US Bureau of Labor Statistics: 1) basic chemicals, which include pigments and dyes, gases, and petrochemicals; 2) synthetic materials such as plastics; 3) agricultural chemicals such as fertilizers and pesticides; 4) painting products; 5) cleaning products, such as soaps and detergents; and 6) other chemicals, which include film products, ink toners, and explosives. Another important sector of the chemical manufacturing industry is the production of pharmaceuticals. Chromium is employed in a variety of chemical processes – often as a catalyst for chemical reactions – and is frequently used for the manufacturing of chromic acid, synthetic dyes, pharmaceuticals and antiseptics, cleaning materials, and toners.
Though the manufacturing of chemicals with chromium is varied, there are several general ways that pollutants can be released into the environment. The first is through wastewater, which can be generated while cleaning equipment, from chemical spills, and from cooling water used during heating procedures. This water often contains chromium, and when not treated or disposed of properly, can carry contaminants into local water supplies. Many chemical processes also require heating, which can release chromium vapor into the air. Chromium pollution created by chemical manufacturing, then, can reach people through residential and drinking water systems, and can also contaminate soil and food. Chemical manufacturing facilities in low- and middle-income countries, where environmental and health regulations may not be as stringent, may cause large amounts of chromium pollution through untreated effluent, with the largest problems, according to Blacksmith’s data, occurring throughout South and Southeast Asia.
Health Effects
Chromium commonly occurs in two forms. Trivalent chromium (chromium III) is a naturally occurring element that is relatively stable and innocuous, and can be found in plants, animals, and soil. Hexavalent chromium (chromium VI) is far more dangerous for humans, and is usually created by anthropogenic causes.
Hexavalent chromium is a toxic human carcinogen that can cause or increase the rates of certain cancers. Inhalation of chromium VI, which occurs most frequently among workers, has been found to cause cancer of the respiratory system. Inhalation of dust contaminated with chromium can also lead to eye damage, ulcerations, swelling, asthmatic bronchitis, and irritation to the throat and nose. More chronic exposure can sometimes cause sores to develop in the nose and can even lead to the formation of holes in the nasal septum.
Ingestion of chromium VI can cause stomach problems, such as ulcers, and can also be damaging for kidney and liver functions. Dermal contact causes a number of skin problems, including rashes, sores, and ulcers.
In addition, several studies have found evidence that chromium accumulation in the body can damage a person’s ability to metabolize iron, which can lead to iron deficiency anemia.
Chemical Manufacturing – Mercury Pollution Mercury has been used in chemical manufacturing processes for many years, as it is a useful catalyst and reagent and can aid in the production of many different substances. Mercury is most commonly used in the manufacturing of pharmaceuticals, cleaning agents, dyes, explosives, and preservatives. Mercury is also used in a variety of devices at some chemical manufacturing facilities, such as thermometers. Mercury can be released during chemical manufacturing much in the same ways as chromium – through effluent and emissions. If not properly treated or contained, wastewater from washing, spills, and cooling – in addition to breaking of thermometers or other products containing elemental mercury, like fluorescent light bulbs – can all lead to mercury contamination of ground and surface water systems. Mercury can also be released through emissions from chemical heating processes.
Mercury has been used in chemical manufacturing processes for many years, as it is a useful catalyst and reagent and can aid in the production of many different substances. Mercury is most commonly used in the manufacturing of pharmaceuticals, cleaning agents, dyes, explosives, and preservatives. Mercury is also used in a variety of devices at some chemical manufacturing facilities, such as thermometers. Mercury can be released during chemical manufacturing much in the same ways as chromium – through effluent and emissions. If not properly treated or contained, wastewater from washing, spills, and cooling – in addition to breaking of thermometers or other products containing elemental mercury, like fluorescent light bulbs – can all lead to mercury contamination of ground and surface water systems. Mercury can also be released through emissions from chemical heating processes.
Mercury pollution from chemical manufacturing can be very dangerous for workers who may come into direct contact with liquid or vapor mercury, and the toxin can also enter drinking water systems, soil, and the food chain. Countries with poor infrastructure and effluent treatment facilities are particularly at risk from wastewater contamination, and Blacksmith estimates that mercury from chemical manufacturing impacts many people in Eastern Europe, Northern Eurasia, and Central Asia.
Health Effects
The health hazards that result from exposure to mercury depend on the level of exposure and the way in which the pollutant enters the body. Inhalation of mercury vapor is particularly hazardous for kidneys, the central nervous system, and the respiratory and cardiovascular systems. Inhalation of mercury vapor has also been found to cause neurobehavioral disorders, such as hand tremor and mental retardation. Exposure to other forms of mercury – and in particular the methylmercury that accumulates in fish – can also lead to problems with the kidneys, lungs, and central nervous system, in addition to arthritis, reproductive problems, loss of memory, psychosis, and in some cases, death. Children exposed to mercury contamination have a higher risk of developmental complications.
Dye Industry – Chromium Pollution
The manufacturing and use of textile dyes is a varied and widespread industry that can either be very large in scale, or can occur in small and sometimes informal facilities. Most dyes get their color from the mixing of different compounds, many of which are toxic in nature. The most common colors associated with chromium use are yellows, oranges, greens, and reds, and these pigments are created by combining various chromium compounds with other elements.
Though there are many different processes that occur in the textile dying industry, a common operation will include pretreatment and bleaching of the material, dyeing in a wet bath, and post-dyeing with fixatives.60 All of these processes create large volumes of wastewater that contain traces of heavy metals from the dyes. If this wastewater is not treated properly, or is dumped directly into nearby water systems, it can contaminate drinking and residential water with large amounts of chromium. Because many dyeing facilities throughout the world are small and sometimes family-run, it is prohibitively expensive to have proper effluent treatment equipment for every one. In countries where environmental and health standards are more relaxed, this can cause a large pollution problem when these smaller facilities dump toxins freely into water systems. Empty dye containers can also cause chromium contamination of water and soil when disposed of improperly.
Health Effects
Chromium commonly occurs in two forms.
Trivalent chromium (chromium III) is a naturally occurring element that
is relatively stable and innocuous, and can be found in plants, animals,
and soil. Hexavalent chromium (chromium VI) is far more dangerous for
humans, and is usually created by anthropogenic causes.
Hexavalent
chromium is a toxic human carcinogen that can cause or increase the
rates of certain cancers. Inhalation of chromium VI, which occurs most
frequently among workers, has been found to cause cancer of the
respiratory system. Inhalation of dust contaminated with chromium can
also lead to eye damage, ulcerations, swelling, asthmatic bronchitis,
and irritation to the throat and nose. More chronic exposure can
sometimes cause sores to develop in the nose and can even lead to the
formation of holes in the nasal septum. 42
Ingestion
of chromium VI can cause stomach problems, such as ulcers, and can also
be damaging for kidney and liver functions. Dermal contact causes a
number of skin problems, including rashes, sores, and ulcers.
In
addition, several studies have found evidence that chromium accumulation
in the body can damage a person’s ability to metabolize iron, which can
lead to iron deficiency anemia.
Industrial Estates – Chromium Pollution Industrial Estates are zoned areas that accommodate grouped industrial facilities. The industry in these planned areas is diverse and the amount of facilities can range from under ten to over one hundred. These areas exist all over the world, with almost 4,500 estimated to be in Asia. Though the industries within these zones can be very diverse, the most common industries that produce chromium contamination are metal processing (chromium coatings are used on a variety of metals to help prevent rust), stainless steel welding, chrome pigment and dye production, and leather tanning.
Industrial Estates are zoned areas that accommodate grouped industrial facilities. The industry in these planned areas is diverse and the amount of facilities can range from under ten to over one hundred. These areas exist all over the world, with almost 4,500 estimated to be in Asia. Though the industries within these zones can be very diverse, the most common industries that produce chromium contamination are metal processing (chromium coatings are used on a variety of metals to help prevent rust), stainless steel welding, chrome pigment and dye production, and leather tanning.  Due to the diverse processes and industries of each industrial estate, chromium pollution from these areas can contaminate water, soil, air, and food. The majority of chromium produced by industrial activities is in the more toxic, hexavalent form. Air pollution from industries that use chromium compounds, especially those that involve heating processes, can be very dangerous for both workers and nearby communities where airborne toxins may settle. Wastewater that is not treated properly can release chromium into water systems, and solid waste from frequent illegal dumping activities can leach contaminants into soil.
Due to the diverse processes and industries of each industrial estate, chromium pollution from these areas can contaminate water, soil, air, and food. The majority of chromium produced by industrial activities is in the more toxic, hexavalent form. Air pollution from industries that use chromium compounds, especially those that involve heating processes, can be very dangerous for both workers and nearby communities where airborne toxins may settle. Wastewater that is not treated properly can release chromium into water systems, and solid waste from frequent illegal dumping activities can leach contaminants into soil.
Health Effects
Chromium commonly occurs in two forms. Trivalent chromium (chromium III) is a naturally occurring element that is relatively stable and innocuous, and can be found in plants, animals, and soil. Hexavalent chromium (chromium VI) is far more dangerous for humans, and is usually created by anthropogenic causes.
Hexavalent chromium is a toxic human carcinogen that can cause or increase the rates of certain cancers. Inhalation of chromium VI, which occurs most frequently among workers, has been found to cause cancer of the respiratory system. Inhalation of dust contaminated with chromium can also lead to eye damage, ulcerations, swelling, asthmatic bronchitis, and irritation to the throat and nose. More chronic exposure can sometimes cause sores to develop in the nose and can even lead to the formation of holes in the nasal septum. 42
Ingestion of chromium VI can cause stomach problems, such as ulcers, and can also be damaging for kidney and liver functions. Dermal contact causes a number of skin problems, including rashes, sores, and ulcers.
In addition, several studies have found evidence that chromium accumulation in the body can damage a person’s ability to metabolize iron, which can lead to iron deficiency anemia.
Industrial and Municipal Dump Sites – Lead Pollution Global production of solid waste, which includes both industrial and municipal garbage, accumulates every day at a staggering rate. Some of the biggest cities in the world, such as Dhaka, Lagos, and Manila, create 5 to 7,000 tons of solid waste every day, and a recent study found that Bangalore produces approximately 1,500 tons per day of municipal waste. In low- and middle-income countries, many cities do not have government-run waste collection, and much of the waste is dumped in large and uncontained areas that are either in the middle of residential areas or right outside of them. Municipal waste can consist of a variety of different substances including food waste, electronics, paper and plastic packaging, glass, etc. Industrial waste is usually created in larger quantities and can contain extremely hazardous materials, such as heavy metals, volatile organic compounds, pesticides, and radionuclides. Lead contamination from dumpsites can come from a countless number of solid waste materials, such as electronics, batteries, paint, and medical supplies.
Global production of solid waste, which includes both industrial and municipal garbage, accumulates every day at a staggering rate. Some of the biggest cities in the world, such as Dhaka, Lagos, and Manila, create 5 to 7,000 tons of solid waste every day, and a recent study found that Bangalore produces approximately 1,500 tons per day of municipal waste. In low- and middle-income countries, many cities do not have government-run waste collection, and much of the waste is dumped in large and uncontained areas that are either in the middle of residential areas or right outside of them. Municipal waste can consist of a variety of different substances including food waste, electronics, paper and plastic packaging, glass, etc. Industrial waste is usually created in larger quantities and can contain extremely hazardous materials, such as heavy metals, volatile organic compounds, pesticides, and radionuclides. Lead contamination from dumpsites can come from a countless number of solid waste materials, such as electronics, batteries, paint, and medical supplies.
The garbage at these dumpsites often produces gas as the material decomposes and rots, and toxic substances can either leach into the soil and contaminate groundwater or can be carried into surface water systems by rain runoff. The amount of toxins released by dumpsites can be tremendously large, with one study finding lead levels in the soil of a former municipal and industrial waste area reaching 10,297 mg/kg – nearly 10 times above the health standard.61 In addition to contaminants entering the environment, many people come into direct contact with lead toxins by scavenging and even sometimes living in the dumpsites. Large and uncontrolled dumpsites can potentially impact hundreds of thousands of people, with some of the largest problems existing in Tanzania, Kenya, Indonesia, and Pakistan.
Health Effects
The health effects of exposure to lead can be both acute and chronic, and the problems caused by lead poisoning are particularly dangerous and severe for children. Acute lead poisoning can happen immediately and is often caused by inhaling large quantities of lead dust or fumes in the air. Chronic lead poisoning, however, occurs over longer periods of time and can result from very low-level, but constant, exposure to lead. Chronic poisoning is far more common than acute exposure and can be caused by persistent inhaling or ingestion of lead, or, over much longer periods, can result in lead accumulation in the bones.
Health problems associated with lead poisoning can include reduced IQ, anemia, neurological damage, physical growth impairments, nerve disorders, pain and aching in muscles and bones, memory loss, kidney disorders, retardation, tiredness and headaches, and lead colic, which impacts the abdomen.19 Severe exposure to high concentrations of lead can lead to dire health risks, including seizures, delirium, coma, and in some cases, death.
Neurological damage is especially pronounced in children suffering from lead exposure, with even small amounts of lead poisoning capable of causing lifelong developmental and cognitive problems. Exposure to lead in utero can also cause birth defects.
Mining and Ore Processing – Arsenic Pollution
Arsenic is mined for use in a variety of different products, including batteries, insect control for livestock, and several types of pesticides. About 2,800 tons of arsenic were also used for the production of lewisite, a chemical weapon agent now destroyed a few years ago under the Chemical Weapons Convention. Nearly 90 % of mined arsenic is used in a compound known as copper chromated arsenate, which is a common wood preservative. In addition, arsenic occurs frequently in the earth’s crust, and is often present in the ore of other commonly mined metals, such as lead, copper, and gold. Arsenic, then, can easily be released into the environment through the mining and ore processing activities of these metals. Waste rock, tailings, and smelting processes at mines where arsenic is present in ore can release the toxin into the environment. One study of arsenic contamination near a gold mining site found that surface waters surrounding the mine had arsenic concentrations of 560 μg/L – 50 times the standard for drinking water.67
As arsenic is an element, it cannot be destroyed. It can easily spread from soil to air or water, and vice versa. Once the toxin is released from the earth’s crust, it can bond with hydrogen and oxygen to become an organic compound, and most arsenic pollution will find its way into soil or water sediment where it can contaminate drinking water and agricultural products. Inhalation, ingestion, and dermal contact with arsenic can all pose human health risks.
Health Effects
Arsenic is known to be a dangerous toxin that can lead to death when large amounts are ingested. Small amounts of arsenic exposure over long periods of time can also lead to numerous health problems, including abnormal heart beat, damage to blood vessels and a decrease of red and white blood cells, nausea and vomiting, and clearly visible irritations of the skin. A common effect of arsenicosis, or arsenic poisoning, is dark patches of skin, corns, or warts on the body. 52
Arsenic is also a documented human carcinogen, and exposure over long periods has been found to cause cancer of the bladder, skin, lungs, kidney, and liver. 53
Mining and Ore Processing – Cadmium Pollution Cadmium is a naturally occurring element in the earth’s crust that commonly occurs as either an oxide, sulfide, or chloride compound and is used in a variety of different industries, including metal plating and for plastic, pigment, and battery production. Cadmium is almost never mined for directly, but rather is obtained as a by-product of the mining and smelting of zinc, copper, and lead ores.68 Cadmium pollution can also be created by the mining of coal, as one study found that there were high levels of cadmium in blood (>0.5 μg/dL) in 85 % of children under the age of six who lived near a coal mine in Turkey.69 Though cadmium can enter the environment in many different ways, smelting and ore processing of zinc is one of the leading anthropogenic causes of cadmium pollution.70 Because cadmium is present in the ores of commonly mined elements, it can be transferred into the environment through waste rock and ore processing tailings. If mines contain smelters at their ore processing facilities, cadmium can also be released into the air during the heating of ore or can remain in the waste slag created by the smelting and refining process.
Cadmium is a naturally occurring element in the earth’s crust that commonly occurs as either an oxide, sulfide, or chloride compound and is used in a variety of different industries, including metal plating and for plastic, pigment, and battery production. Cadmium is almost never mined for directly, but rather is obtained as a by-product of the mining and smelting of zinc, copper, and lead ores.68 Cadmium pollution can also be created by the mining of coal, as one study found that there were high levels of cadmium in blood (>0.5 μg/dL) in 85 % of children under the age of six who lived near a coal mine in Turkey.69 Though cadmium can enter the environment in many different ways, smelting and ore processing of zinc is one of the leading anthropogenic causes of cadmium pollution.70 Because cadmium is present in the ores of commonly mined elements, it can be transferred into the environment through waste rock and ore processing tailings. If mines contain smelters at their ore processing facilities, cadmium can also be released into the air during the heating of ore or can remain in the waste slag created by the smelting and refining process.
Once airborne, cadmium can travel easily as dust or vapor, and often contaminates soil and food when it settles back to the ground. The ingestion of food contaminated with cadmium is a fairly common route of exposure. Cadmium compounds in mining waste rock and tailings can leach into water and soil and contaminate drinking wells and water sources used for bathing and irrigation. Workers in the mines can be exposed to high levels of cadmium dust and vapor when proper precautions are not taken. Cadmium is a very hazardous material that is a known human carcinogen. Inhalation and oral exposure to cadmium can cause chronic lung and kidney disorders, and in extreme cases, exposure may cause cancer.
Mining and Ore Processing – Cyanide Pollution
Unlike the other common pollutants caused by mining and ore processing, cyanide is not formed in the earth’s crust, but is instead created by various types of algae, bacteria, fungi, and plants.71 Cyanide contamination is commonly associated with the mining of metals, particularly gold, since it can bond with the desired metals and help isolate them from their ore. Cyanide use is normally part of the ore processing phase, and the most common methods are vat-leaching and heap-leaching. Vat-leaching consists of mixing crushed ore with a cyanide compound in a vat where it will form a new compound with the metal and can aid in isolating desired material from the unwanted waste rock. The typical amount of cyanide used in this process can range from 300 to 500 mg/L.72 For heap-leaching, mounds of crushed ore are either placed in large pits or, preferably, on impermeable material. Cyanide is then poured, dripped, or sprayed onto the mounds, and it forms compounds with the metals in the ore as it leaches through the pile. Though this practice is relatively cheap, it only yields about 50 to 75 % of the gold content from the ore. 73
Mining and ore processing activities that use cyanide can lead to toxic environmental contamination through water and soil. The leaching processes, if done improperly or without the necessary oversight, can create large amounts of wastewater that often contains trace amounts of cyanide. The toxin can also remain in the tailings left behind from the ore processing, where it can leach into soil and groundwater systems, or can enter the air as dust. Cyanide is a highly toxic substance, and acute exposure can lead to heart and brain problems, and in some cases, can cause death.74 Chronic exposure to cyanide through inhalation, ingestion, or dermal contact can cause problems with breathing, headaches, thyroid enlargement, chest pain, seizures, and skin irritation or sores.75
Product Manufacturing – Lead Pollution (especially from plating, electronics manufacture, and battery manufacture) The product manufacturing industry – which includes hundreds of different manufacturing materials and processes – is a major part of the global economy and is responsible for many of the goods that people use on a daily basis. Lead is commonly used in many products due to its ability to protect against radiation and sound waves, its capacity to be recharged, and its resistance to water corrosion. Lead compounds are used for metal finishing and in the production of automobile parts, batteries, tires, and a wide range of electronic equipment, such as air conditioners, refrigerators, televisions, and computers. Many of the production processes for these products require water use, heating, and chemical reactions, all of which can release lead into the environment.
The product manufacturing industry – which includes hundreds of different manufacturing materials and processes – is a major part of the global economy and is responsible for many of the goods that people use on a daily basis. Lead is commonly used in many products due to its ability to protect against radiation and sound waves, its capacity to be recharged, and its resistance to water corrosion. Lead compounds are used for metal finishing and in the production of automobile parts, batteries, tires, and a wide range of electronic equipment, such as air conditioners, refrigerators, televisions, and computers. Many of the production processes for these products require water use, heating, and chemical reactions, all of which can release lead into the environment.
Though lead contamination can occur during the processing of all of the aforementioned products, batteries are the main producer of toxic lead pollution. The manufacturing of batteries – which are predominantly used in cars but can also be used in other common products, such as computers – can release large amounts of lead dust and fumes into the air during plating and assembly processes, which can be harmful to workers and the surrounding environment if the dust is not properly contained. Wastewater from the cleaning and cooling processes during manufacturing can also carry lead into nearby water systems. The full life-cycle of a battery must also be considered, which would include the lead emissions of used battery recycling. A recent report estimated that the manufacturing and recycling of a projected one billion computer batteries in low- and middle-income countries through 2015 would create about 1.2 to 2.3 million tons of lead pollution – “between four and seven times the weight of the Empire State Building.” 76
Health Effects
The health effects of exposure to lead can
be both acute and chronic, and the problems caused by lead poisoning are
particularly dangerous and severe for children. Acute lead poisoning
can happen immediately and is often caused by inhaling large quantities
of lead dust or fumes in the air. Chronic lead poisoning, however,
occurs over longer periods of time and can result from very low-level,
but constant, exposure to lead. Chronic poisoning is far more common
than acute exposure and can be caused by persistent inhaling or
ingestion of lead, or, over much longer periods, can result in lead
accumulation in the bones.
Health problems associated with lead
poisoning can include reduced IQ, anemia, neurological damage, physical
growth impairments, nerve disorders, pain and aching in muscles and
bones, memory loss, kidney disorders, retardation, tiredness and
headaches, and lead colic, which impacts the abdomen.19
Severe exposure to high concentrations of lead can lead to dire health
risks, including seizures, delirium, coma, and in some cases, death.
Neurological
damage is especially pronounced in children suffering from lead
exposure, with even small amounts of lead poisoning capable of causing
lifelong developmental and cognitive problems. Exposure to lead in utero
can also cause birth defects.
Uranium Mining and Ore Processing – Radionuclide Pollution Uranium, which is a radionuclide, is widely used as a source of fuel for nuclear power plants. Mining for this element occurs all throughout the world, and uranium is typically extracted through open-pit mining and in-situ leaching, which consists of injecting acid liquids or sodium biocarbonate into an ore deposit in order to bring valuable ore to the surface. Once the uranium ore is obtained, it must be ground into smaller pieces and combined with chemicals, such as sulfuric acid, in order to acquire pure uranium. The concentration of uranium in ore is very low, only accounting for 0.1 to 0.2 % of the ore content, which means that very large amounts of waste rock are created during the mining process. In addition, the processing of the ore leaves enormous amounts of toxic sludge that often contains radioactive materials, including left over uranium, thorium-232, and their decay product radium, which can contain as much as 85 % of the initial radioactivity of the ore.77
Uranium, which is a radionuclide, is widely used as a source of fuel for nuclear power plants. Mining for this element occurs all throughout the world, and uranium is typically extracted through open-pit mining and in-situ leaching, which consists of injecting acid liquids or sodium biocarbonate into an ore deposit in order to bring valuable ore to the surface. Once the uranium ore is obtained, it must be ground into smaller pieces and combined with chemicals, such as sulfuric acid, in order to acquire pure uranium. The concentration of uranium in ore is very low, only accounting for 0.1 to 0.2 % of the ore content, which means that very large amounts of waste rock are created during the mining process. In addition, the processing of the ore leaves enormous amounts of toxic sludge that often contains radioactive materials, including left over uranium, thorium-232, and their decay product radium, which can contain as much as 85 % of the initial radioactivity of the ore.77
Radionuclides are naturally occurring elements that are radioactive, meaning that they have atoms with unstable nuclei. As elements or materials decay, they will emit radiation up to an end point in the decay process. Some materials decay quickly, but some, like uranium, can continue to be radioactive for millions of years. During the decay, different types of radioactivity with different health effects can be generated. Uranium mining in low- and middle-income countries is frequently lacking in oversight and regulatory practices, which can allow large amounts of toxic waste rock and tailings to contaminate the soil, air, water, and food of surrounding areas. People who live near these mines may also be exposed to radiation. Uranium radionuclides can cause damage to kidneys and to the genetic code, which can often impact fetal development. Other radionuclides, such as radon, can lead to leukemia and decreases in white blood cell counts. Several of the areas where uranium mining is conducted on the largest scale are in Central Asia, Namibia, Russia, and Niger, and two of the largest examples of the dangers of uranium mining and enrichment leading to radionuclide contamination are the Chernobyl site in Ukraine and the Fukushima nuclear power plant in Japan.
A Note on Oil Production Pollution from oil use and production is generally outside of the scope of Blacksmith’s work, due to the globally pervasive nature of this industry and number of sites impacted by oil pollution. Basically, inclusion of this industry within Blacksmith’s work would overwhelm our current resources and lead to poorer understanding of the impact of other industries. However, in view of the thousands of sites contaminated by the petrochemical industry, often in highly populated areas, and the many health impacts to which oil and petrochemical product exposure can lead, Blacksmith believes that, if the data existed, the petrochemical industry would probably be included as one of the top ten pollution problems. For this reason, it is important to discuss the dangers and health effects posed by this industry, as it likely impacts millions of people throughout the world.
Pollution from oil use and production is generally outside of the scope of Blacksmith’s work, due to the globally pervasive nature of this industry and number of sites impacted by oil pollution. Basically, inclusion of this industry within Blacksmith’s work would overwhelm our current resources and lead to poorer understanding of the impact of other industries. However, in view of the thousands of sites contaminated by the petrochemical industry, often in highly populated areas, and the many health impacts to which oil and petrochemical product exposure can lead, Blacksmith believes that, if the data existed, the petrochemical industry would probably be included as one of the top ten pollution problems. For this reason, it is important to discuss the dangers and health effects posed by this industry, as it likely impacts millions of people throughout the world.
The oil industry consists of many different processes, all of which have the potential to cause extreme environmental damage and health problems. The processes include drilling for crude oil, refining of oil and production of petrochemicals, and transportation of refined products, all of which can lead to harmful spills, leaks, and waste.
Once crude oil is extracted from the ground it must be chemically processed into commercial products. Oil refineries process crude material into over 2,500 products, including many different types of fuel, lubricants, asphalt, paraffin wax, tar, and petrochemicals.78 Oil processing can be a fairly complicated procedure, with many operations that could potentially release large amounts of toxins and other pollutants into the environment. Volatile chemicals that are produced through the refining process can enter the atmosphere and can cause high levels of ambient air pollution. If done improperly or without appropriate equipment and oversight, the refining of oil can lead to spills of oil and wastewater that contaminate water systems and soil. Given the waste produced by oil refining – in general, processing one ton of crude oil can produce 3.5 to 5 cubic meters of wastewater and 3 to 5 kilograms of sludge and solid waste – proper containment and treatment of this material is very important to prevent widespread environmental damage. 79
Pollutants from oil refining and spills can contaminate the air, water, soil, and a large variety of food products. People who live near oil refineries or oil spill locations are at risk of inhaling or ingesting toxic materials, and those who are exposed are at risk of developing skin lesions, digestive and respiratory problems, and cancer. Several of the most harmful contaminants that people may be exposed to from oil pollution are volatile aromatic organic compounds such as xylene, benzene, and toluene. Volatile aliphatic compounds such as hexane and heptane are also key pollutants. Due to the demand for oil and its production on such a mass scale throughout the world, the oil production and refining industry poses a large risk to environmental and human health – especially when proper precautions and standards are not taken.
Conclusion Toxic pollution caused by mining and industrial processes throughout the world poses an enormous health risk to affected populations. Pollutants from these processes, as well as from some naturally occurring sources, such as arsenic in groundwater, are responsible for a significant amount of deaths and diseases every year, particularly in low- and middle-income countries. This report has developed estimates of health impacts from ten of the most prevalent types and sources of toxic pollution, in countries where Blacksmith’s data show significant occurrence of these sources.
Toxic pollution caused by mining and industrial processes throughout the world poses an enormous health risk to affected populations. Pollutants from these processes, as well as from some naturally occurring sources, such as arsenic in groundwater, are responsible for a significant amount of deaths and diseases every year, particularly in low- and middle-income countries. This report has developed estimates of health impacts from ten of the most prevalent types and sources of toxic pollution, in countries where Blacksmith’s data show significant occurrence of these sources.
The report shows that, where studies on the health effects of toxics exist and basic assessment data from contaminated sites is collected, it is possible to quantify the level and severity of disease caused by toxic pollution. Using Blacksmith’s site-specific data, this report has calculated DALYs associated with health impacts caused by exposure to a pollutant at one representative site for each of the top ten issues. These DALYs are large, and illustrate the enormous disease burden of death and disability associated with toxic pollution exposure.
It is important to note key qualifications related to the health impact estimates:
- These estimates are based only on acute effects from exposure through the primary pathway, typically ingestion or inhalation. The human health impact from exposure to toxins at levels above health standards is relatively well studied; however, relatively less is known about disease and other health impacts due to lower exposures or the cumulative impact from exposure pathways.
- As stated earlier in the report, it is difficult to estimate the exposed population around a toxic contamination site. The calculations are based on the best available demographic data that site assessors could collect from local and national government records, aerial photographs, and personal observation.
- DALY calculations are based on limited sampling of soil, water, or human biological indicators (e.g., blood or breath tests.) More testing would provide improved estimates of exposure of the impacted population.
- Blacksmith’s database of contaminated sites is the most comprehensive such database now existing for most low- and middle-income countries. However, Blacksmith is aware that there are many more sites in almost all such countries that have not been assessed.
Because of the above limitations, it is not possible to develop specific estimates of DALYs for specific toxic pollutants for an entire country, no less the world. However, in order to show the likely magnitude of health impacts of toxic pollution and be able to compare this health burden to other health burdens, a range of exposed populations has been estimated for five countries for various specific pollutants, related to the top ten toxic pollution causes discussed in this report. To develop the range, Blacksmith used information in our database about the total number of sites identified in a country with a specific toxic pollutant and the number of exposed people at those sites to estimate a potentially exposed population for the entire country.
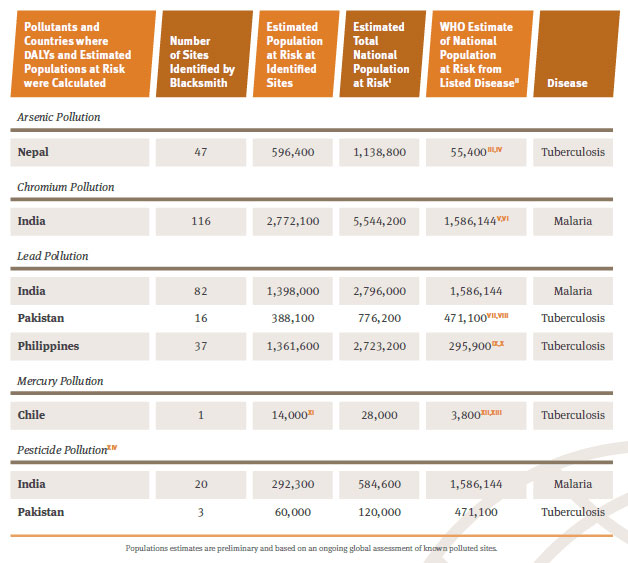
[i]: Blacksmith estimates that our site assessments have captured approximately 50% of existing sites in each country. The total national population at risk is an estimate of the actual number of people at risk at all existing sites in the country, including those not yet identified and assessed by Blacksmith.
[ii]: Because the populations at risk from pollution exposure include both deaths and incidence of disease, these numbers also illustrate the at risk population for other diseases using death and incidence rate. All estimates are based on WHO data from 2008, 2009 and 2010. Because the incidence rates only account for cases of a disease measured in one particular year, these numbers do not reflect repeat cases of a disease or cases that have not gone into remission. For this reason, the number of people at risk from these health problems at any given time is likely significantly larger.
[iii]: World Health Organization. “TB Country Profile: Nepal.” Accessed on October 17, 2011. Available at: http://www.who.int/gho/countries/npl/country_profiles/en/index.html.
[iv]: World Health Organization. “Global Health Observatory Data Repository: Mortality and Burden of Disease: Disease and industry country estimates 2008: By sex.” April, 2011. Available at: http://apps.who.int/ghodata/?vid=10011.
[v]: World Health Organization. “Malaria Country Profile: India.” 2010. Available at: http://www.who.int/gho/countries/ind/country_profiles/en/index.html.
[vi]: World Health Organization. “Global Health Observatory Data Repository: Mortality and Burden of Disease: Disease and industry country estimates 2008: By sex.” April, 2011. Available at: http://apps.who.int/ghodata/?vid=10011.
[vii]: World Health Organization. “TB Country Profile: Pakistan.” Accessed on October 17, 2011. Available at: http://www.who.int/gho/countries/pak/country_profiles/en/index.html.
[viii]: World Health Organization. “Global Health Observatory Data Repository: Mortality and Burden of Disease: Disease and industry country estimates 2008: By sex.” April, 2011. Available at: http://apps.who.int/ghodata/?vid=10011.
[ix]: World Health Organization. “TB Country Profile: Philippines.” Accessed on October 17, 2011. Available at: http://www.who.int/gho/countries/phl/country_profiles/en/index.html.
[x]: World Health Organization. “Global Health Observatory Data Repository: Mortality and Burden of Disease: Disease and industry country estimates 2008: By sex.” April, 2011. Available at: http://apps.who.int/ghodata/?vid=10011.
[xi]: The estimated at risk population from exposure to mercury in Chile from Blacksmith research alone is quite low. To date, Blacksmith has only assessed one mine site in Chile where mercury is the key pollutant, and this presents a large underrepresentation of Chileans who are likely impacted. The mercury contamination from the Blacksmith site is caused by a large copper and gold mine—both of which are very prevalent throughout Chile. A rough and conservative estimate of the number of actual gold and copper mines in Chile is approximately 100 (http://www.ame.com.au/Countries/Cu/Chile.htm, and http://www.ame.com.au/Countries/Au/Chile.htm).
[xii]: World Health Organization. “Tuberculosis Profile: Chile.” October 17, 2011. Available at: https://extranet.who.int/sree/Reports?op=Replet&name=/WHO_HQ_Reports/G2/PROD/EXT/TBCountryProfile&ISO2=cl&outtype=pdf.
[xiii]: World Health Organization. “Global Health Observatory Data Repository: Mortality and Burden of Disease: Disease and industry country estimates 2008: By sex.” April, 2011. Available at: http://apps.who.int/ghodata/?vid=10011.
[xiv]: It is very difficult to estimate the actual population that is at risk from exposure to pesticides in these countries (and in almost every case), as large percentages of each country are involved in agricultural processes. Over 50% of the labor force in India works in agriculture, for example (CIA World Factbook: India. Available at: https://www.cia.gov/library/publications/the-world-factbook/geos/in.html). Thus, the estimated impacted population from Blacksmith’s site assessments under represents the actual numbers of people at risk. The table below presents the results of our country-wide specific pollutant exposure estimating process. The table then contrasts the exposed population associated with the pollutants discussed in the top ten issues to the at-risk or exposed populations for other various diseases in the same country, based on WHO data. These comparisons illustrate that toxic pollution potentially causes just as large (if not larger) of a disease burden as other well known and documented health problems in low- and middle-income countries.
The table below presents the results of our country-wide specific pollutant exposure estimating process. The table then contrasts the exposed population associated with the pollutants discussed in the top ten issues to the at-risk or exposed populations for other various diseases in the same country, based on WHO data. These comparisons illustrate that toxic pollution potentially causes just as large (if not larger) of a disease burden as other well known and documented health problems in low- and middle-income countries.
In view of the estimated health risk and burden caused by toxic pollution exposure worldwide, there is a clear need to address these problems. Significant resources are dedicated (appropriately) to addressing the health burden presented by disease diseases with similar or even lower health risks and burdens to those presented by toxic sites. However, resources to address toxic contamination are, in many countries, very limited. Clearly, countries with toxic contamination have the primary responsibility for remediating the sites and addressing the health impacts. However, there is also a need for the international community to help fund clean up efforts, provide technical support, and help with on-the-ground training to educate people about the dangers of toxins. Such support would be similar to help provided for various diseases and is needed due to the limited financial and/or technical resources in many low- or middle-income countries.
Blacksmith continues to identify and assess sites contaminated by toxic pollution in order to better understand the global scope of this issue and reduce the significant human health risks it causes.
Addressing the health impacts of toxic pollution in low- and middle-income countries is not an impossible undertaking. Though the number is large, there are a discreet number of sites, and remediation can often be done for a moderate cost. Blacksmith Institute and Green Cross Switzerland have demonstrated successful clean up and education projects in severely contaminated areas that are both cost-effective and replicable. There are well-documented and researched solutions for many of the pollution problems discussed in this report. Given proper funding and resources, many of these solutions and programs can be implemented worldwide. Addressing pollution is not only a question of ethics, but is also far more cost-effective than the long-term social and economic costs of pollution. It is our intention to continue identifying and addressing these problems throughout the world and to show the benefits of preventative measures in order to ensure adequate and equal health for the world’s population.
Footnotes:
[10]: “New Basel guidelines to improve recycling of old batteries.” United Nations Environment Programme. May 22, 2002. Available at: http://www.unep.org/Documents.Multilingual/Default.asp?DocumentID=248&ArticleID=3069&I=en.
[42]:“Health Effects of Hexavalent Chromium.” OSHA Factsheet. Occupational Safety and Health Administration. U.S. Department of Labor. July 2006. Available at: http://www.osha.gov/OshDoc/data_General_Facts/hexavalent_chromium.pdf.
[52]: “ToxFAQs For Arsenic.” Department for Health and Human
Services: Agency for Toxic Substances and Disease Registry. August 2007.
Available at: http://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=19&tid=3.
[53]: “Water-related diseases: Arsenicosis.” World Health Organization: Water Sanitation and Health. Accessed on September 1, 2011. Available at: http://www.who.int/water_sanitation_health/diseases/arsenicosis/en/.
[58]:“Cadmium Fact Sheet.” Environmental Protection Agency. Accessed on September 21, 2011. Available at: http://www.epa.gov/osw/hazard/wastemin/minimize/factshts/cadmium.pdf.
[60]: “Title III Section 313 Release Reporting Guidance: Estimating Chemical Releases from Textile Dyeing.” United States Environmental Protection Agency. February 1988. Available at: http://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=10003BI6.txt.
[61]:Carr, Ramona, et al. “Identification and mapping of heavy metal pollution in soils of a sports ground in Galway City, Ireland, using a portable XRF analyzer and GIS.” Environmental Geochemistry and Health, Vol. 30, No. 1 (July 2007): 45-52.
[69]: Yapici, Gulcin, et al. “Lead and cadmium exposure in children living around a coal-mining area in Yata?an, Turkey.” Toxicology and Industrial Health, Vol. 22, No. 8 (2006): 357-362.
[70]: “Pollution Prevention and Abatement Handbook: Cadmium.” International Finance Corporation, World Bank Group. July 1998. Available at: http://www.ifc.org/ifcext/enviro.nsf/attachmentsbytitle/p_ppah_pguicadmium/$file/handbookcadmium.pdf.
[71]:“Toxic Substances Portal: Cyanide.” Agency for Toxic Substances and Disease Registry. Accessed on September 21, 2011. Available at: http://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=19.
[72]:“Use of Cyanide in the Gold Industry.” International Cyanide Management Code for the Gold Mining Industry. Accessed on September 21, 2011. Available at: http://www.cyanidecode.org/cyanide_use.php.
[73]:“Use of Cyanide in the Gold Industry.” International Cyanide Management Code for the Gold Mining Industry. Accessed on September 21, 2011. Available at: http://www.cyanidecode.org/cyanide_use.php.
[74]:“Division of Toxicology and Environmental Medicine ToxFAQs: Cyanide.” Agency for Toxic Substances and Disease Registry. July 2006. Available at: http://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=71&tid=19.
[75]:“Division of Toxicology and Environmental Medicine ToxFAQs: Cyanide.” Agency for Toxic Substances and Disease Registry. July 2006. Available at: http://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=71&tid=19.
[76]:Cherry, Christopher R. and Perry Gottesfeld. “Plans to distribute the next billion computers by 2015 creates lead pollution risk.” Journal of Cleaner Production, Vol. 17 (2009): 1620-1628.

-
Artisanal Gold Mining
Mercury Pollution
-
Industrial Estates
Lead Pollution
-
Agricultural Production
Pesticide Pollution
-
Lead Smelting
Lead Pollution
-
Tannery Operations
Chromium Pollution
-
Mining and Ore Processing
Mercury Pollution
-
Mining and Ore Processing
Lead Pollution
-
Lead-Acid Battery Recycling
Lead Pollution
-
Arsenic in Groundwater
Arsenic Pollution
-
Pesticide Manufacturing and Storage
Pesticide Pollution
-
The Rest of the Toxic Twenty



